top of page
Radiation Dosimetry System
Next-generation skin dosimetry technology for safer cancer treatment
Each year, over 20 million people are diagnosed with cancer, and half undergo radiation therapy.
Yet, clinicians still rely on outdated and time-consuming dosimetry tools that are prone to inaccuracy.
The result? Delayed feedback, patient discomfort, and inconsistent treatment quality.
The MOSkin™ project set out to solve this by creating a simple, precise, and immediate radiation monitoring system that transforms patient safety.

The MOSkin™ system is redefining how radiation dose is measured during therapy.
Developed by Electrogenics Laboratories together with Genesys Electronics Design, Design + Industry (Capgemini), and the University of Wollongong, it transforms pioneering semiconductor research into a reliable clinical tool.
Built on custom MOSFET technology, the lightweight, translucent sensor adheres directly to the patient’s skin, providing instant and highly accurate radiation readings without affecting imaging or treatment.
The MOSkin system brings together three key components for seamless operation:
-
Disposable Skin Sensor: flexible and translucent, measuring radiation dose directly on the patient.
-
Hub Unit: collects and transmits readings from up to four sensors simultaneously.
-
Reader App: displays real-time dose data and manages patient information.
Together, they create a connected ecosystem that delivers immediate, reliable, and traceable radiation data, empowering clinicians with insights when it matters most.

Genesys’ Engineering Leadership

As the lead engineering partner, Genesys led the transition of MOSkin™ from research prototype to regulatory-ready medical device.
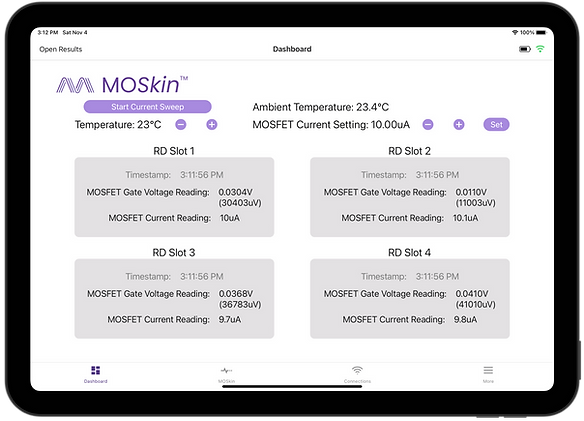
Our engineers conducted extensive semiconductor packaging investigations, developed precision low-current measurement and multiplexing electronics, and built the embedded firmware and mobile application that manages the system, guides the user through the process and displays radiation dose information (key features include authentication/sign-in pages, user account management, and connection status).
With rigorous risk analysis and ISO 13485 compliance planning, Genesys ensured the entire platform was designed for manufacturability, traceability, and long-term reliability.

Let’s create technology that makes healthcare safer and smarter.
"Let's build something extraordinary together."
bottom of page
.png)