MEDICAL TECHNOLOGIES
Our Medical Device Certification
Genesys is certified in the ISO 13485 quality standard for electronics hardware and software development for Medical Devices. This means you can be assured that our designed will meet regulatory requirements globally.

Integrated MedTech Design Services
Genesys provides Product Realisation services for medical technologies with electronics and software. In addition to the core electronics/software design services, a division of the company called
Genesys Medical Devices provides "whole-of-product" development and manufacturing services.
Services by Genesys Electronics Design include:
-
Regulatory compliance technical support for electronics and software
-
Medical App Development
including SAMD or device integrated Apps.
Services of Genesys Medical Devices and its strategic partners include:
-
Commercialisation planning
-
Intellectual property
-
Design and Development (whole-of-product)
-
Industrial Design
-
Usability Engineering
-
Regulatory strategy and compliance
-
Clinical Trials
-
Quality management systems
-
Sterilisation and packaging
-
Electronics manufacturing
-
Contract (box build) manufacturing, inc. supply chain
-
Project Management
Download our whitepaper on What You Need To Know BEFORE Developing Electronic Medical Devices.
Introducing the Genesys Medical
Wireless Wearable Sensor platform
Genesys has developed a wireless Bluetooth connected platform for logging sensor data and wirelessly egressing it to a companion mobile App.
The platform provides all the "under-the-hood" hardware and software enablers while making it easy to add your specific or custom sensors to the common/standard in-built ones we provide. This allows you to focus on your end user requirements.
Our baseboard and firmware enablers have been developed under medical device design controls which will accelerate your pathway to regulatory compliance.


Medical Mobile App Development
We develop medical Apps optimised for integration with medical devices, delivering the user interface, application logic and all the support facilities.
By building your App on top of our template, all of the under-the-hood facilities around navigation, user management, gathering device data, graphical data display, cybersecurity and data integrity come out-of-the box.
By using our modularised software and templates developed under medical device design controls and addressing issues of using an uncontrolled mobile device platform, we make your regulatory compliance tasks much easier.

Our Case Studies
TruScreen offers the latest technology in cervical screening, providing real-time, accurate detection of pre-cancerous and cancerous cervical cells to help improve the health and wellbeing of women around the world.

Cervical Cancer Screening

CT Injection System

The Salient CT Injector injects contrast materials such as radio-opaque dye for patients undergoing CT scans. It is cordless, compact and mobile with a user friendly touch screen interface.


Why Choose Genesys?
SPECIALISTS IN ELECTRONICS FOR MEDTECH
With more than 15 specialist engineers in MedTech certified software and hardware design, we are the Go To product development firm in Australia
INTEGRATED DESIGN & COMPLIANCE
Regulatory consultants will tell you WHAT you need to do. We tell you HOW it should be done – fully integrating the technical implementation with all necessary compliance steps.
IEC 62304 COMPLIANT SOFTWARE LIBRARY
Genesys has developed a library of hardware and software modules that are already compliant with the IEC 62304 medical software standard. This proven library delivers unparalled reliability.
ONE PASS TO PRODUCTION - GUARANTEED!
We are the only company in Australia to guarantee that we will deliver a working prototype first time, or we will make good at our own expense. This eliminates your risk of cost over-runs.
Our Contribution
Genesys is proud to organise and sponsor the NSW Active MedTech Community.
The purpose of this community is saving and improving lives by making it easier to develop medical devices with electronics and software.
For more information visit the community at:

NSW Health Director of Enterprise & International Partnerships Ann O'Neill (lower right) launched the NSW Active MedTech Community at Venture Cafe on 17 Nov 2019.
What you need to know BEFORE
Developing Electronic Medical Devices
Genesys has developed several principles that underpin successful medical device development:
-
Charting the optimal path to market: There are many complex business and technical decisions made in your product development journey. Considerations include how the product will be sponsored and when to start the documentation process required for certification. Of particular importance is understanding how to balance the demands of the minimum viable product approach and the need for intelligent road-mapping of the product architecture to minimise whole-of-life costs.
-
De-risking your project: Entrepreneurs need to manage their learning curve and apply due diligence in setting up your systems to manage the many risks your project faces. Focus on outcomes rather than technology. Embrace standards as a way of minimising technical risk and the workload required to identify the hazards involved with outstanding risks.
-
Minimising whole-of-life costs: Being smart about your minimum viable product can greatly reduce overall product development costs. Genesys develops a product road map with an architecture that maximises early features and minimises the cost of adding them later.
-
Going beyond superficiality in compliance: Many regulatory consultants will tell you what you need to do, but not how you can go about doing it. Startups need to understand the devil-in-the-detail of regulations and standards.
-
Establish quality systems that actually help: Go beyond tick-box quality to help you establish systems that actually help run your business better. Plan for compliance from the start
We apply all these philosophies internally on the work we do to deliver your product.
To find out more about our approach to medical quality, download our whitepaper on What you need to know BEFORE developing electronic medical devices.
Our Medical Technologies
Genesys has a library of technologies that apply in a range of health industry sectors. See our white labelling service to have the following example applications adapted for your business, with full ISO 13485 certification.
IEC 60601-1 COMPLIANT HARDWARE
Our embedded electronics modules are fully compliant with requirements of IEC 60601-1 for safety and essential performance in medical devices.
IEC 62304 MEDICAL SOFTWARE
Genesys has a library of software modules compliant with the IEC 62304 medical software standard. This enable us to develop embedded firmware or standalone medical software.
PATIENT
SAFETY
Our embedded electronics modules are fully compliant with requirements of IEC 60601-1 for safety and essential performance in medical devices.
MEDICAL SENSING EXPERTISE
We have experience in sensing a range of medical parameters including physiological waveforms, body temperature, body movement, EEG, ECG and SP02.
HEALTH DATA MANAGEMENT
Our technologies can analyse data in the device or securely deliver it to the cloud. We handle the stringent requirement for patient privacy and data integrity using encryption and checksum technologies.
Medical Engineering Standards
Genesys is expert in the design of a product to the following standards:
-
Quality Systems ( ISO 13485): This is the overarching quality standard system that guides product development for medical devices. Everyone involved in the product's development needs to be certified to the standard or audited by someone that is. Genesys is ISO 13485 certified for medical device electronics hardware and software development.
-
Risk management and hazard analysis (ISO 14971): This standard covers the application of risk management to medical devices, particularly as it relates to patient and operator safety. It primarily involves identification of hazards and mitigating risk. Safety by design and other protective measures are key risk management features, as well as the use of information, labeling, warnings and training resources.
-
Safety and essential performance (IEC 60601-1): While much of this series of technical standards for medical electrical equipment relates to safety, the requirements for essential performance cut to the heart of the device's ability to carry out the medical functions effectively without creating unacceptable risk. Determining the risks associated with the essential performance of a device can be much more challenging than determining basic electrical safety requirements which are usually set out in other standards.
-
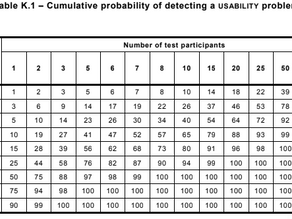
Usability engineering (IEC 62366): This standard covers the application of usability engineering to medical devices. Usability covers both the potential for error in the device's use and the ease of using the device for its intended purpose. Unique to every device, the scope of usability covers everything from the physical form of the device and its mechanical operation to clarity of labeling and graphical interfaces.
-
Software development (IEC62304): This standard specifies the requirements for firmware and software in medical devices at every stage of the product's life cycle. Of critical importance is the traceability of user requirements. Note that standalone software can be classed as a medical device if it has a medical purpose.
For more information view the full list of standards we regularly deal with.
.png)